Risk of emerging marine biotoxins in British shellfish – Pinnatoxin
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Background
1. The Food Standards Agency (FSA) is considering the current advice and monitoring programme for marine biotoxins and whether there is a need to update or change existing legislative standards.
2. The main purpose of this work is to identify any emerging marine biotoxins in UK waters, including a consideration of the potential increases in occurrence with increasing temperatures due to climate change. The views of the Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) are sought on whether any of these marine biotoxins would pose a risk to human health. A scoping paper providing an overview of emerging biotoxins will be brought to the Committee in the Autumn, as well as a discussion paper on Pectenotoxins.
3. The current paper provides information and data on the risks to human health associated with consumption of shellfish from UK waters, in relation to the class of emerging marine biotoxin known as pinnatoxins (PnTXs). These are currently unregulated and unmonitored in shellfish from UK waters. The paper therefore considers the toxicological database for PnTXs and whether there is a need to include PnTXs in the current FSA England and Wales biotoxin monitoring programme. Considerations have also been given to the likelihood of pinnatoxins becoming more prevalent due to climate change and rising sea water temperatures around the UK. The paper draws on previous evaluations by other bodies and authorities. A literature search was also conducted, and relevant papers up to the 1st March 2023 have been included. Details of the literature search can be found in Annex A.
Introduction
4. Pinnatoxins (PnTXs), first isolated from the bivalve mollusc Pinna attenuata in the South China Sea (Zheng et al., 1990) are a family of lipophilic biotoxins of which, to date, eight analogues (PnTX-A - PnTX-H) have been identified. These toxins belong to the group of cyclic imines (CI) and share similarities to other CIs in terms of their chemical structures and molecular targets.
Previous Evaluations
5. In 2006, regulatory monitoring of shellfish for lipophilic toxins in the French Mediterranean showed that mussel extracts collected at the Ingril lagoon induced an atypical neurotoxic effect in mouse bioassays. In 2011, researchers identified Pinnatoxin G (PnTX-G) as the toxic compound and analysis of mussel samples collected from 2009 to 2012 revealed regular occurrences of PnTX-G at levels sufficient to account for the toxicity in the mouse bioassays (Hess et al., 2013). Levels of PnTX-G regularly exceeded 40 µg/ kg in whole flesh, with levels of PnTX-G reaching 1244 μg/kg of shellfish (National Institute for Ocean Science in France (Ifremer), 2010; ANSES, 2019). Cultures of microalgae Vulcanodinium rugosum, previously isolated from Ingril lagoon, confirmed that this algae produced PnTX-G, and was most likely responsible for PnTX-G contamination of local shellfish (Hess et al., 2013).
6. Despite the acute toxicity observed in mouse bioassays, to date, no human intoxication has been reported (Delcourt et al., 2019).
FAO/IOC/WHO 2004
7. In 2004, the Food and Agricultural Organization of the United Nations (FAO), International Oceanographic Commission of UNESCO (IOC) and World Health Organisation (WHO), published a joint expert consultation which considered what scientific advice could be provided to enable the establishment of maximum levels of shellfish toxins in shellfish as well as any guidance on methods of analysis for each toxin group. As part of their assessment, they examined available toxicity data for the CI group of toxins including gymnodimine, spirolides, pinnatoxins, prorocentrolide and spirocentrimine.
8. The report noted that there were no data on the oral toxicity of pinnatoxins and the expert group considered the database insufficient to establish an acute reference dose (RfD) or tolerable daily intake (TDI) for any of the PnTXs or CIs considered (FAO, 2004).
EFSA 2010
9. In 2010, the EFSA Panel on Contaminants in the Food Chain (CONTAM) published their Scientific Opinion on the risk of human consumption of CI, specifically spirolides, gymnodimines, pinnatoxins and pteriatoxins, in shellfish.
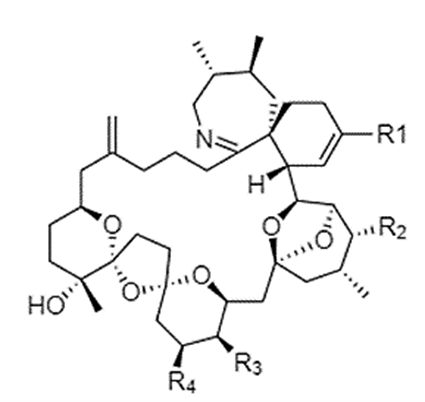
10. EFSA noted that acute toxicity studies for PnTXs with intraperitoneal (i.p.) injection in mice demonstrated potent toxicity for every PnTX-analogue tested (PnTX-A, -B, -C, -D, -E, -F and -G), with PnTX-F being the most toxic analogue tested (LD50 16 μg/kg bodyweight (b.w.)). Oral LD50 values were only available in one study from a mixture of PnTX-E and -F in algal extract (estimated 10 μg PnTX/mg), giving LD50 values of 23 μg/kg b.w. by oral gavage. These were the lowest oral LD50s reported for any CIs in the EFSA assessment.
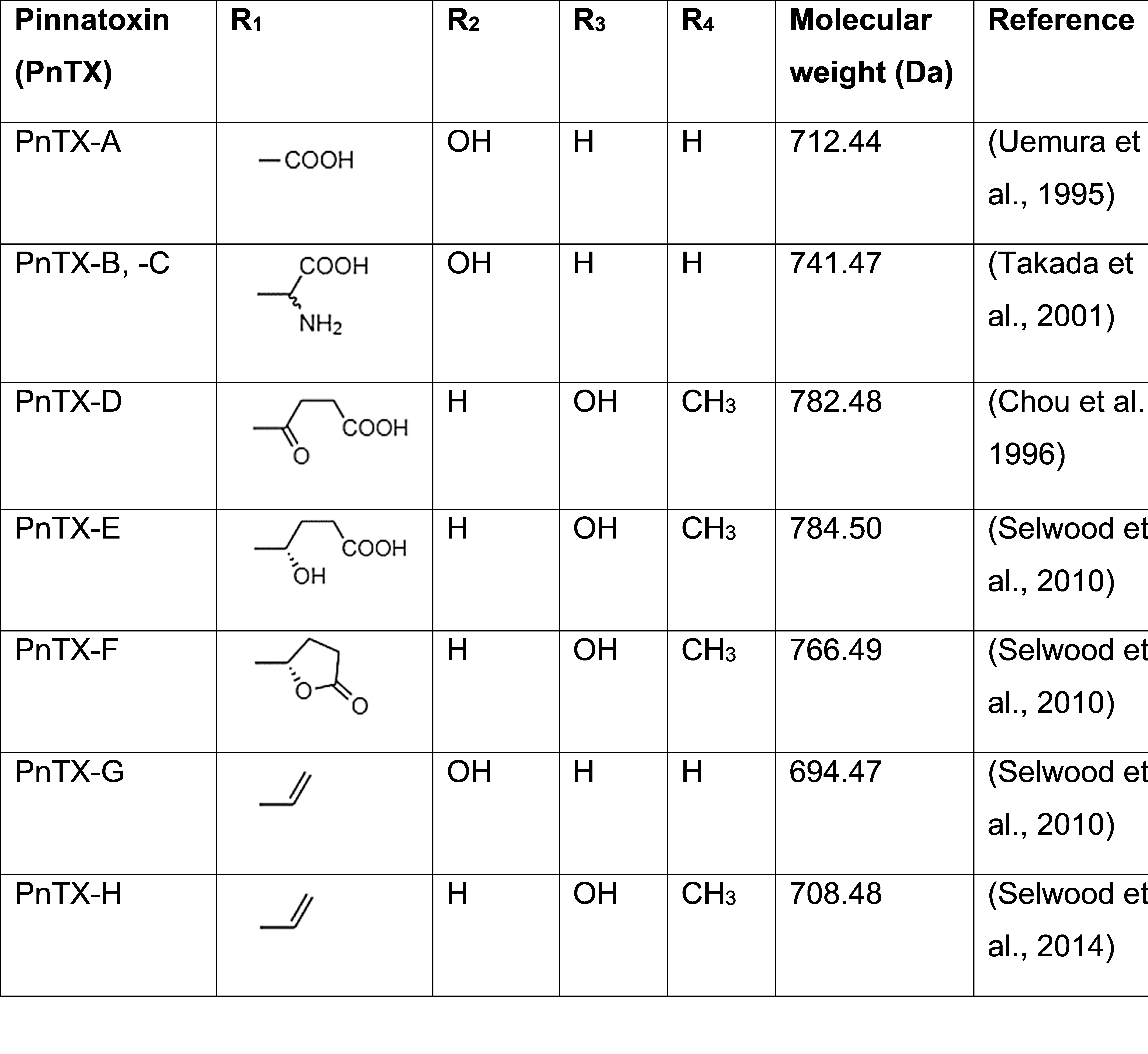
11. EFSA noted that although CIs, including PnTXs, have been known to occur in microalgae and shellfish, to date no poisoning events in humans have been linked to these toxins. They concluded that because no long-term studies on the groups of CIs in experimental animals were available, it was not possible to establish a TDI. While EFSA considered that an acute RfD should be established for the different groups of CIs, they were unable to do so for PnTXs due to the lack of adequate quantitative data on the acute oral toxicity i.e., no-observed-adverse-effect levels (NOAELs) (EFSA, 2010).
CEFAS 2014
12. In 2014, the Centre for Environment, Fisheries and Aquaculture Science (CEFAS), the Scottish Association of Marine Science (SAMS) and Agri-Food and Biosciences Institute (AFBI) carried out a literature review and survey on new and emerging marine biotoxins in shellfish in UK waters. The report sought to identify suitable existing testing methods or potential new methods to support the development of a risk-based monitoring programme for emerging marine biotoxins in UK shellfish harvesting waters, in response to the recent amendment to the EU hygiene legislation.
13. The report recommended monitoring PnTXs in UK waters through a screening method based on appropriate analytical methods, such as Liquid Chromatography with Mass Spectrometry (LC-MS) but noted that this was dependent on availability of appropriate analytical standards. If implementation of a quantitative method was not possible, efforts should be made to develop and validate a screening method for PnTXs.
14. The report further recommended Vulcanodinium sp. to be included on the toxic species list as the causative organism of PnTXs. but also due to its presence in Norwegian waters, and therefore the possibility for it to become established in UK waters. However, the report noted that inclusion in the toxic species list would require a microscopy monitoring programme and more detailed taxonomic information (CEFAS, 2014).
ANSES 2019
15. In 2012, the National Institute for Ocean Science in France (Ifremer), reported the presence of PnTXs in mussels from the Ingril lagoon in the south of France. In 2019, the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) provided their assessment of health risks associated with PnTX in shellfish along with recommendations for monitoring.
16. ANSES concluded that based on the available acute toxicological data, which showed neurotoxicity of PnTXs in mice, there was sufficient evidence for a potential hazard to humans. ANSES also carried out a risk assessment specifically on PnTX‑G, due to it being detected in shellfish from the Ingril lagoon. Based on an acute oral toxicity study in mice, ANSES proposed a provisional acute benchmark value of 0.13 µg PnTX-G/kg bw. Based on the extrapolation from a serving size of 400 g of shellfish and a body weight of 70 kg, they recommended that a concentration of 23 µg PnTX-G/kg of total meat should not be exceeded in shellfish meant for human consumption. ANSES further applied available consumption data to estimate dietary exposure from shellfish and concluded that it would be possible for the benchmark value to be exceeded in some cases.
17. ANSES recommended that screening for PnTXs should be included as part of the official surveillance of lipophilic toxins in shellfish and that monitoring of the dinoflagellate V. rugosum should be set up (ANSES, 2019).
Hazard identification
Chemical characterisation
18. PnTXs are characterised by their polyether macrocyclic structure which contains a 6,7-spiro ring, a 5,6-bicyclo ring and a 6,5,6-trispiroketal polyether ring involving 14 chiral centres in the primary structure (Figure 1).
Figure 1: Chemical structures of pinnatoxin.
19. PnTX-analogues vary in the length and functionality of their cyclohexenyl side chains, as well as the methylation and hydroxylation on their various ring systems (Table 1).
Table 1: Chemical structures and molecular weight of pinnatoxins.
20. PnTXs are water soluble due to their amphoteric nature (Zendong et al., 2014) but can also be detected during the mouse bioassay due to their lipophilic properties.
21. LC-MS analysis of pacific oysters and razor fish extracts in 2010 revealed the presence of a variety of metabolites and intermediates of PnTXs in shellfish tissue. Reactive epoxides and oxidized or hydrolysed forms of PnTX-G, plausible intermediate states to PnTX-A, -B and -C, indicate that PnTXs undergo biotransformation in shellfish. No information was available regarding the levels or toxicity of these intermediates, but they are likely to be short lived based on the reactive nature of the intermediate functional group (Selwood et al., 2010). Shellfish have also been found to contain several acyl derivatives of PnTXs due to metabolic transformation within the shellfish tissue (Aráoz et al., 2020; McCarron et al., 2012).
Toxicity
Toxicokinetics
22. A permeability assay with human intestinal epithelium monolayers of Caco-2 cells found PnTX-G weakly to moderately permeated monolayers of Caco-2 cells (~20%). The majority of passage occurred in the first two hours of exposure and stabilized thereafter, regardless of initial PnTX-G concentration (11.5, 23.1 or 46.1 nM) or whether the PnTX-G was applied as a crude extract or in purified form. Permeability of PnTX-A was inconclusive as researchers noted technical issues that occurred in an apparent dose-dependent manner with PnTX-A (ANSES-Universite de Trieste-Cnr, 2014).
23. Servent et al. (2021) exposed rats to 3 μg/kg radiolabelled [3H]-PnTX-G by oral gavage or intravenous (i.v.) administration, followed by digital autoradiography. Blood samples collected between 2 and 15 minutes after injection showed rapid clearance from the blood, with a half-life of 13 ± 4 minutes, and an elimination constant of 0.06 ± 0.02 per minute. Quantification of β-radioactivity in blood and organs using liquid scintillation analysis showed accumulation of the compound in the liver and small intestine (14.6 ± 4.4% and 17.5 ± 6.0% of the administered dose, respectively) with some accumulation (<5%) in kidneys and lungs. Urine, sampled 15 minutes after injection, seemed the primary route of elimination containing 3.6 ± 0.6% of the administered dose, whereas faeces elimination after 4 hours accounted for only 0.04 ± 0.02% of the administered dose.
24. Rats exposed by oral gavage to 100 μg/kg of [3H]-PnTX-G in phosphate buffered saline also showed rapid accumulation in the liver, kidney, and spleen, with biodistribution of the toxin within 15 minutes, similar to that after i.v. injection. The authors observed radioactivity in various brain regions such as the hippocampus and superficial grey layer of the superior colliculus (SuG) as well as some skeletal muscles such as the extensor digitorum longus (EDL) and gastrocnemius muscles (Servent et al., 2021).
25. Radiolabelled PnTX-G was detected in the placenta of pregnant rats (19 days post-fertilization) 30 minutes after injection, corresponding to 0.73% of the injected dose, as well as in the embryos (0.45%). PnTX-G was observed most strongly in embryo tongue, liver, hindlimb and forelimb skeletal muscles, and several areas of the central nervous system, demonstrating the toxin's ability to cross the placental barrier and to distribute into various tissues/organs. In addition to PnTX-G, minor metabolites were also detected, corresponding to 20% of the injected toxin in brain extract and 45% in liver extracts, indicating a possible role of this organ in PnTX-G metabolization (Servent et al., 2021).
In vitro toxicity
Cytotoxicity
26. Crude extracts of V. rugosum showed significant cytotoxicity in Neuro2A and KB cells in vitro. IC50 values of 0.38 µg/mL and 0.19 µg/mL were reported for Neuro2A cells after 24 hours of incubation and for KB cells after 72 hours of incubation, respectively. By contrast, no inhibition of cell viability was observed with PnTX-G on the Neuro2A cell lines exposed to concentrations as high as 32 ng/mL as well as on KB cells exposed to up to 400 ng/mL. It has been suggested that cytotoxicity detected in crude extracts could be due to other compounds, such as nakijiquinone A, N-carboxy-methyl-smenospongine, stachybotrin A, or from one of several major compounds unidentified in the extract (Geiger et al., 2013). In other studies, portimine, a compound produced by V. rugosum, an induce apoptosis and is highly cytotoxic when incubated with cultured cells (Cuddihy et al., 2016).
In vivo toxicity
27. To date, there are no confirmed cases of poisoning in humans linked to PnTXs, and no human in vivo data exists as to the toxicity of PnTXs when consumed or PnTX-contaminated foods.
Toxicity of V. rugosum extracts in mice
28. Extracts from cultures of V. rugosum strains containing both PnTX-E and -F showed acute toxicity in the mouse bioassay with LD50 values of 1.3 and 2.3 mg/kg administrated by i.p. injection or oral gavage, respectively and 5.95 mg/kg by administration in feed (Rhodes et al., 2010). Limited information was available on the exact composition of the extract and therefore other possible components were present.
Toxicity of isolated and purified pinnatoxins in mice
29. The onset of toxic effects in mice occurs rapidly, with symptoms appearing a few minutes after oral or i.p administration. This is in agreement with other CIs which belong to the fast-acting toxins group (EFSA, 2010). At lethal concentrations, mice rapidly become immobile; their respiration rates decline until respiration ceases entirely. Death occurs 15-50 minutes after administration (ANSES-Universite de Trieste-Cnr, 2014; Munday et al., 2012; Selwood et al., 2010). Two studies suggested that these symptoms could be preceded by an initial phase of hyperactivity or tremors and jumps (ANSES-Universite de Trieste-Cnr, 2014; Selwood et al., 2010). One of the studies also noted slight exophthalmia (Selwood et al., 2010). In studies with toxic, but sub-lethal doses (~75% of the LD50), activity also decreased and respiration rate declined with mice being lethargic and showing piloerection. However, their appearance and behaviour normalised again after 1 to 1.5 hours and mice were fully recovered after 2 to 3 hours. Animals remained normal throughout the remainder of the subsequent observation period, and no abnormalities were recorded at necropsy, with organ weights being within the normal range (Munday et al., 2012; Selwood et al., 2010).
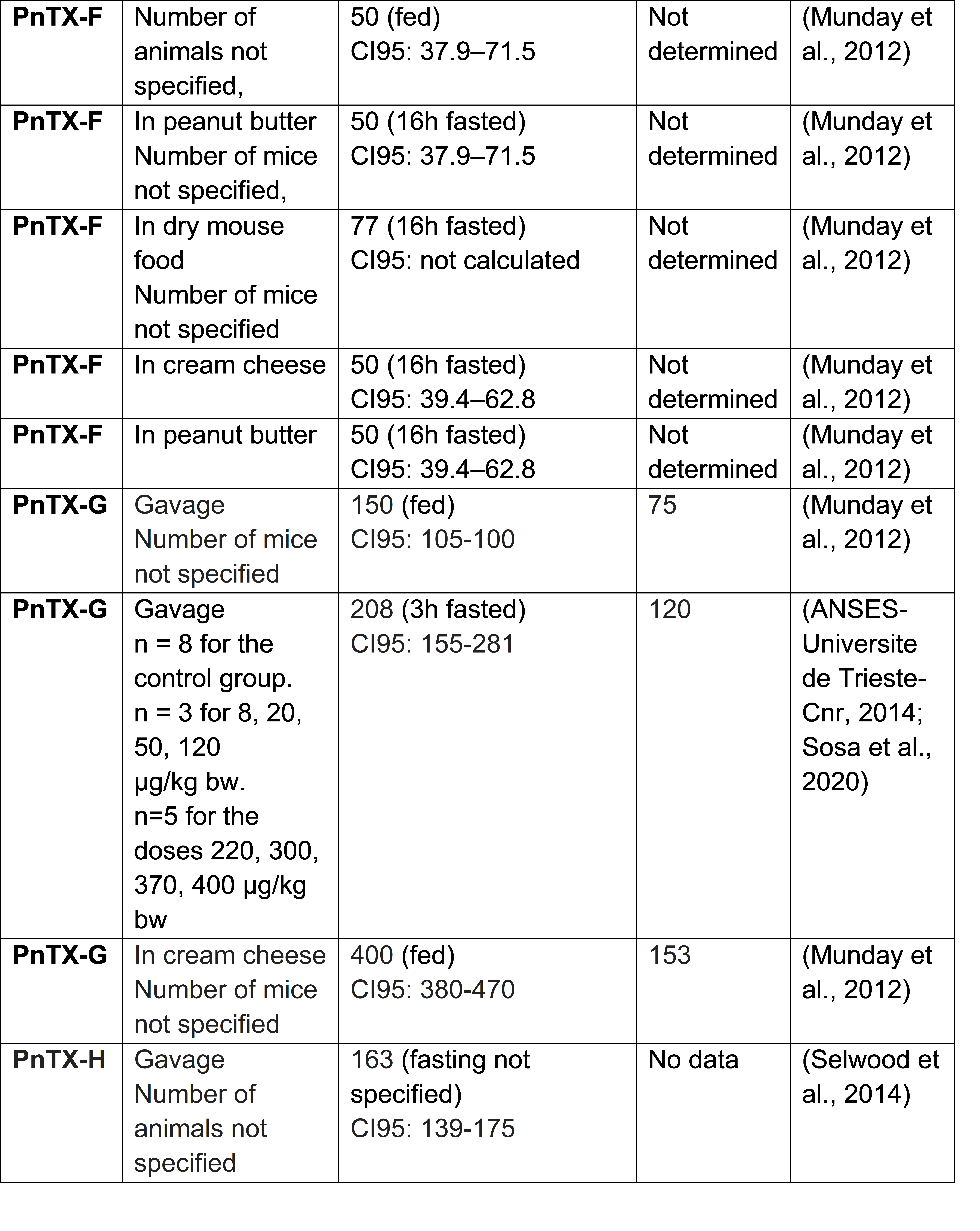
30. Administration of increasing doses of synthetic or purified PnTXs to mice by oral gavage produced LD50s of 2800, 25, 150 and 163 μg/kg bw, for PnTX-E, -F, -G and H, respectively. The maximum tolerated dose (i.e. the dose at which neither mortality or adverse effects were observed), correlated strongly with the LD50s (Table 2; ANSES-Universite de Trieste-Cnr, 2014; Munday et al., 2012; Selwood et al., 2014; Sosa et al., 2020). Munday et al. (2012), also compared acute toxicity via oral gavage with exposures via foodstuff (e.g., cream cheese) to represent a “voluntary consumption” scenario, as would be the case for human consumption. Oral gavage produced a lower LD50 than exposure via foodstuffs (25 vs 50 μg/kg bw for PnTX-F, 150 vs 400 μg/kg bw for PnTX-G). Based on the available studies the toxicity of the PnTX-analogues after oral administration was suggested as follows: PnTX-F > PnTX-G ~ PnTX-H ≫ PnTX-E (Table 2; ANSES-Universite de Trieste-Cnr, 2014; Munday et al., 2012; Selwood et al., 2014; Sosa et al., 2020). No data regarding the oral toxicity of PnTX-A, PnTX-B, PnTX-C, or PnTX-D were available.
Table 2: Acute in vivo toxicity in mice of PnTXs after oral administration (Adapted from ANSES, 2019).
*LD50: defined as the amount of the ingested substance that killed 50 percent of the test sample. **CI95: 95% confidence interval. ***MTD: Maximum tolerated dose (dose at which neither mortality was observed, nor clinical signs were evident).
31. Exposure of mice to PnTX-A, -E, -F, -G and -H via i.p injection resulted in LD50s of 115, 45-57, 13-16, 48-50 and 67 μg/kg bw, respectively (Table 3). Based on the available studies, the toxicity of the PnTX-analogues after i.p. administration was suggested as follows: PnTX-F > PnTX-G > PnTX-E > PnTX-H > PnTX-A (Arnich et al., 2020; Munday et al., 2012; Selwood et al., 2014, 2010). No data regarding the toxicity of the PnTX-B, -C, or -D analogues after i.p. administration were available.
Table 3: Acute in vivo toxicity in mice of PnTXs after i.p. administration (Adapted from ANSES, 2019).
*LD50: defined as the amount of the ingested substance that killed 50 percent of the test sample. **CI95: 95% confidence interval. ***MTD: Maximum tolerated dose (dose at which neither mortality was observed, nor clinical signs were evident).
32. Exposures to the crude extract of V. rugosum, resulted in a ratio of 1:1.75 for oral : i.p. injection (Rhodes et al., 2011a), while for PnTX-H, the oral LD50 value was only 2.4 times that of the LD50 by i.p. injection (Selwood et al., 2010). For PnTX-F and PnTX-G, the ratios between oral and i.p. injections were 2.0 and 3.1, respectively (Munday et al., 2012). By comparison, other CIs such as gymnodimine and the spirolides were 8 to 23 times less toxic by gavage than by injection (Munday et al., 2012). Interestingly, this feature does not seem to be the case for PnTX-E which was 48 times less toxic by oral gavage than by i.p. injection (Munday et al., 2012).
33. Only limited data were available for acute toxicity of other PnTX-analogues. Uemura et al. (1995) reported an LD99 for PnTX-A and PnTX-B of 180 μg/kg bw and 22 μg/kg bw respectively, but no information on the dosing, species, number of animals or method of administration were available. Similarly, Chou et al. (1996) reported the isolation and characterisation of PnTX-D with an LD99 of 0.4 mg/kg in mice after i.p. administration but no information on the dosing or number of animals was provided. Takada et al. (2001) reported an LD99 of 22 μg/kg for isolated and purified PnTX-B and -C in a 1:1 mixture.
Clinical reports of Dermatitis after exposure to V. rugosum
34. A report by Moreira-González et al. (2021) has linked a V. rugosum bloom to 60 cases of acute dermatitis at two recreational beaches in Cienfuegos Bay, Cuba. Individuals who had prolonged contact with the bloom suffered acute dermal irritation. Most patients (79.2%) were children and had to be treated with antibiotics; some required >5-day hospitalization. LC-MS/MS analysis confirmed the presence of portimine, PnTX-F and PnTX-E in the seawater (Moreira-González et al., 2021).
Genotoxicity
35. A study by Geiger et al. (2013) demonstrated that crude extracts of V. rugosum up to 5 µg/mL induce a significant, dose-dependent increase in Ki-67 positive cells, a marker of cell cycle arrest, in Caco-2 cultures 48 h after exposure. This was accompanied by a significant increase in γH2AX immunofluorescence, a marker for DNA damage. In contrast, exposure of Caco-2 cells with up to 5 µg/mL of purified PnTX-G resulted in no increase in the markers for cell cycle arrest or DNA damage.
Mode of Action
Molecular targets of Pinnatoxin
Neuronal type nicotinic acetylcholine receptors
36. CI toxins interact with the major neuronal nicotinic acetylcholine receptors (nAChRs) and pinnatoxins have been shown to be potent inhibitors of human nAChRs in vitro (Molgó et al., 2017).
37. Ex vivo, two-electrode voltage-clamp experiments on Xenopus oocytes expressing the human α7 and α4β2 nAChR demonstrated that PnTX-A, -F, and -G inhibited acetylcholine (ACh)-evoked currents at low or sub nanomolar affinities, with PnTX A and -G display a strong discrimination for human α7 over α4β2 nAChRs (Araoz et al., 2011; Bourne et al., 2015; Hellyer et al., 2015). In one studies, PnTX-A was 300-times less potent against α4β2 than the α7 nAChRs receptor (Araoz et al., 2011). Hence, it has been suggested that PnTXs may discriminate between nAChRs based on their subunit composition.
38. These findings are also supported by competition binding assays, where PnTXs displace [125I]-α-bungarotoxin and/or [3H]-epibatidine from its nAChRs binding site at human α7, α3β2 and α4β2 receptor. Again in these experiments, PnTX-E, -F and -G showed higher affinity for α7 (Ki: 0.025-0.065 nM) over α4β2 nAChRs (Ki: 3-11 nM) (Araoz et al., 2011; Bourne et al., 2015; Hellyer et al., 2015; Molgó et al., 2017). A full list of the known activities of PnTX-analogues against nAChRs are detailed in Annex B.
39. From the available data, the general affinities of PnTXs against nAChRs can be prioritised as the following: nAChRs α7 >> α3β2 ≥ α4β2. However, it is less clear from the data if there is a priority in terms of PnTX-analogues affinity for nAChRs.
40. Servent et al. (2021) examined the molecular specificity of PnTX-G in rat tissue sections in the absence or presence of various specific antagonists of nAChR subtypes. Methyllycaconitine (MLA), an α7 nAChR antagonist, reduced [3H] PnTX G, particularly in hippocampal and hypothalamus regions, indicating toxin-α7 receptor interactions in this region. In contrast, A-85380, a selective binder of the α4β2 receptor subtype, was ineffective in reducing the binding of PnTX-G in hippocampus and hypothalamus regions, but significantly decreased the [3H] PnTX G binding in the SuG compared to MLA, suggesting a prevalence of β2-selective nAChRs in this area, and in particular the α4β2 subtype.
Muscle-type nicotinic acetylcholine receptors
41. Ex Vivo two-electrode voltage-clamp experiments on Xenopus oocytes expressing the muscle-type nAChRs, α12β1γδ, demonstrated that that PnTX-A, and -G inhibit ACh-evoked currents at 5.5 and 3.8 nM respectively (Araoz et al., 2011; Bourne et al., 2015).
42. In radiolabel binding assays, PnTXs show potent inhibition against muscle-type nAChRs. PnTX-A, -E, -F and -G produced concentration-dependent inhibition of [125I]-α-bungarotoxin binding to muscle-type nAChRs in either nAChR-enriched membranes from Torpedo californica or rat muscle microsomal membrane preparation, with IC50s in the low to sub nanomolar range (Araoz et al., 2011; Bourne et al., 2015; Hellyer et al., 2015).
43. In tissue sections of rat embryo radiolabelled with PnTX-G, αC-conotoxin PrXA (0.25 μM), a ligand that selectively interacts with muscle-type nAChR, induced a large decrease in PnTX-G binding, which was particularly notable in peripheral organs such as forelimb skeletal muscles. These results were interpreted as supporting the presence of toxin-muscle-type nAChRs interaction in situ. In contrast, MLA (100 nM), a specific antagonist for neuronal-type nAChRs α7, displaced PnTx G labelling in peripheral tissues only very weakly, highlighting the specificity of the toxin-muscle-type nAChRs interaction (Servent et al., 2021).
44. Fluorescently conjugated PnTX-F applied to muscle sections of thy1-YFP-H transgenic mice, which express yellow fluorescent protein (YFP) in motor nerves, demonstrate PnTX-F labelling of the neuromuscular endplate regions. This binding was inhibited by pre-exposure of muscle sections to native i.e. non-fluorescent, PnTX-F or the nicotinic antagonist α-bungarotoxin indicating direct binding of PnTX-F with nicotinic receptors at the neuromuscular junction endplates (Hellyer et al., 2014).
Functional studies of pinnatoxin
45. To establish the mode of action of the acute respiratory effects observed after administration in vivo, PnTXs were also evaluated in isolated rat hemidiaphragms. Hellyer et al., (2011) found that compound muscle action potentials (CMAPs) were significant reduced by crude extract of PnTX-E/PnTX-F at concentrations of 470 nM as well as purified PnTX-F at concentrations of 260-520 nM, without affecting the twitch response evoked by direct muscle stimulation.
46. Further studies by Hellyer et al. (2013), carried out in phrenic nerve-hemidiaphragm preparations, found PnTX-E, -F and -G blocked neuromuscular transmission, with IC50 values of 53.9, 11.3 nM and 19.1 nM, respectively. Onset of neuromuscular block was rapid at high concentrations, with a complete elimination of the twitch response within 15 to 20 minutes at concentrations above 100 nM (PnTX-F and -G) or 250 nM (PnTX-E). KCl-induced muscle contractions were unaffected after exposure to PnTX-E, -F and -G indicating a lack of direct myotoxic action of PnTXs, as well as a lack of a direct effect on the contractile machinery of the muscle fibres, consistent with a site of action at the neuromuscular synapses.
47. Electrophysiological recordings showed that PnTX-F and -G reduced or completely blocked spontaneous miniature endplate potentials amplitude without any effect on miniature endplate potentials frequency or resting membrane potential, indicative of a postsynaptic mechanism of action (MoA). This was proposed as consistent with a mechanism of PnTXs blocking the interaction of ACh quantal release with endplate nAChRs. As a result, endplate potentials are no longer reaching the threshold for opening voltage-gated sodium channels in muscle fibres, triggering muscle action potentials and twitch responses (Hellyer et al., 2013).
48. Local injections of PnTXs in live animals showed potent inhibitory neuromuscular effects. Benoit et al. (2019) found that intramuscular injections containing various concentrations of either PnTX-A (0.54-5.44 nmol/kg, n = 8 mice) or PnTX-G (1.60-3.20 nmol/kg, n = 18 mice) resulted i a dose-dependent reversible decrease of CMAP amplitude in caudal motor nerve response (at the base of the tail) in live anaesthetized female mice. The doses required to block 50% of the CMAP maximal amplitude were similar between analogues, 3.1 and 2.7 nmol/kg for PnTX-A and PnTX-G, respectively.
49. In the same study, PnTX-A (2.8–84 nM) and PnTX-G (2.5–40 nM) were applied to isolated peroneal nerve-EDL muscle preparations. The toxins reversibly inhibited the amplitude of muscle twitches evoked by nerve stimulation in a time and concentration-dependent manner. PnTX-G had a stronger effect than PnTX-A, inhibiting nerve-evoked twitch responses at concentrations as low as 2.5 nM. However, the onset of the block was about two-times slower with PnTX-G compared to PnTX-A. The IC50 of the peak muscle twitch amplitude were 27.7 nM for PnTX-A, and 11.3 nM for PnTX-G (Benoit et al., 2019).
Health Based Guidance Values
50. In 2019, ANSES carried out a literature review focusing on the available toxicity data for PnTXs. The assessment was limited to the potential risk of PnTX-G, based on the high levels of the toxin detected in French Mediterranean lagoons from 2010 – 2012. They chose as their key study the 2014 Universite de Trieste study which examined acute oral toxicity study in mice with purified PnTX G.
51. To derive a critical dose, two different approaches were employed a) a maximum tolerated dose (MTD) approach with a concentration of 120 µg PnTX-G/kg bw, based on the maximum dose at which no adverse effect is observed in the chosen parameters after toxin administration and b) fitting the available toxicity data using benchmark dose modelling software (PROAST Web software) to derive a benchmark dose lower bound threshold (BMDL95%) value of 69.1 µg PnTX-G/kg bw.
52. Uncertainty factors (UF) were applied to both approaches. An overall UF of 900 was applied to the MTD of 120 µg PnTX-G/kg bw, based on the default UF of 10 for inter-species variability, 10 for inter-individual variability and an additional UF of 3 for insufficient data and 3 to take into account the severity and pattern of the dose-response curve. An overall UF of 525 was applied to the BMDL of 69.1 µg PnTX-G/kg bw, based on an allometric adjustment factor of 7 for the mouse, and UFs of 2.5 for the toxicodynamic component, 10 for inter-individual variability, and 3 for insufficient data.
53. The resulting provisional acute benchmark value was 0.13 µg PnTX-G/kg bw, regardless of the approach used (MTD or BMDL). Given the uncertainties in the assessment ANSES applied an overall confidence level of moderate to the provisional acute benchmark value. ANSES noted that this confidence level was provisional and may be reassessed when new acute oral toxicity data become available.
54. ANSES was unable to assess the long-term effects of PnTX-G due to the lack of data on repeated oral administration with purified PnTX-G. However, by extrapolation from a serving size of 400 g of shellfish and applying a body weight of 70 kg, ANSES recommended that a concentration of 23 µg PnTX-G/kg of total meat not to be exceeded in shellfish.
55. No derivations of HBGVs for PnTX-G or any PnTX analogue by other authorities have been found in the literature.
Occurrence and exposure
56. In 1995, PnTX-A was isolated from the mother-of-pearl Pinna muricata harvested in the Japanese island of Okinawa (Uemura et al., 1995). PnTX-B, -C and -D were identified soon after from P. muricata in Japan (Chou et al., 1996; Takada et al., 2001). Early detection and identification methods were not practical for routine surveillance, but the development of standards and LC-MS methods for pinnatoxins have allowed researchers and surveillance bodies much greater ability to monitor the occurrence of these toxins from seawater and shellfish, and quantify their levels (Selwood et al., 2010).
Occurence in European shellfish
57. The first report of PnTX-G in Europe was in Norwegian shellfish. Repeated sampling suggested that levels of PnTX-G varied significantly based on the location and time of sampling; concentrations of PnTX-G were detected in 69% of the 166 mussel Norwegian samples analysed, with levels as high as 115 µg/kg (Rundberget et al., 2011).
58. After the discovery of PnTX-G in Norway, a retrospective analysis of French surveillance samples from shellfish production areas from 2009 to 2012 found significant contamination with PnTXs. The concentrations varied greatly depending on the year, with maximum annual concentrations of 261, 1244, 568 and 652 µg/kg of total mussel meat for 2009, 2010, 2011 and 2012, respectively. The concentrations of PnTX-G measured in mussels from the Ingril lagoon are the highest reported in the world to date (Hess et al., 2013).
59. In 2018, France established the EMERGTOX scheme for monitoring the emergence of marine biotoxins and found PnTXs to be consistently present in Ingril, Le Scoré in Brittany and the Diana lagoon in Corsica (mainly PnTX-G but also PnTX-A). The Ingril lagoon remains the location with the highest reported concentrations detected every month, varying from 40 to 2614 μg PnTX-G/kg and 6 to 32 μg PnTX-A/kg (measured from digestive gland). The levels found at Le Scoré and Diana are generally lower, with maximum levels around 10 μg PnTX-G/kg of digestive gland (ANSES, 2019).
60. The most consistent published reports of PnTX-contaminated shellfish have come from raw and processed shellfish samples from Northern Spain. PnTX-G has been detected in shellfish from Catalonian, Galician, Atlantic and Cantabrian coastlines with levels of PnTX-G ranging from 0.4 to 60 μg/kg. The most common source were mussels (M. galloprovincialis), but also clams (R. decussatus) and oysters (O. edulis) (García-Altares et al., 2014; Lamas et al., 2019; Moreiras et al., 2020; Otero et al., 2019; Rambla-Alegre et al., 2018; Varriale et al., 2021). Since 2018, PnTX-G has also been detected in raw and processed shellfish samples from Slovenia, Italy, Scotland, Northern Ireland, Ireland, Italy, and Portugal (Aráoz et al., 2020; Rambla-Alegre et al., 2018; Varriale et al., 2021).
61. Where compared in the same study, mussels (M. galloprovincialis) display higher levels of contamination compared to other shellfish species harvested in the same waters. Rambla-Alegre et al., 2018 found processed mussels have a higher frequency of detection for PnTX-G (30%) than clams (2%), consistent with findings in fresh samples (24% mussels and 6% clams). This result is consistent with findings from Ingril Lagoon in France where concentrations of PnTX-G in fresh mussels were consistently higher than those observed in clams (Mean Ratio = 7.6 (Hess et al., 2013).
62. Simultaneous sampling of both whole flesh and digestive glands of mussels indicated that the fraction of PnTXs accumulating in the digestive gland (DG) appeared higher than the levels in whole flesh (WF) (Mean Ratio = 2.95 DG/WF), although lower than is reported for other lipophilic biotoxins, such as azaspiracids and okadeic acid (DG/WF Ratio = 5.2 and 5.5 respectively, Hess et al., 2005; McCarron et al., 2008). Hence, it has been suggested that other mechanisms than direct ingestion of micro-algae might play a role in the accumulation of PnTX-G in mussels (Hess et al., 2013).
63. Table 4 provides an overview of the occurence of PnTXs in European waters and outside Europe.
Table 4: Overview of occurrence of PnTXs in contaminated shellfish from European waters, and outside Europe.
|
Country |
Sample Location |
Shellfish |
Pinnatoxins detected (Conc.) |
Notes |
Ref. |
|
European reports |
European reports |
European reports |
European reports |
European reports |
European reports |
|
France
|
Ingril Lagoon |
Clams (Venerupis decussata) |
PnTX-G (17-95 μg/kg) |
PnTX-A also detected but was 2% compared to PnTx-G |
(Hess et al., 2013)
|
|
France
|
Ingril Lagoon |
Mussels (Mytilus galloprovincialis) |
PnTX-G (37-1244 μg/kg) |
NA |
(Hess et al., 2013)
|
|
France
|
10 coastal sites |
Mussels (Mytilus galloprovincialis) |
PnTX-G (0-7 μg/kg) |
NA |
(Hess et al., 2013)
|
|
France
|
Fish market in Paris |
Clam, Cockle, Oyster, Mussel |
Trace amounts of PnTX-G (<0.1μg/kg) |
NA |
(Aráoz et al., 2020) |
|
Spain
|
Processed seafood samples |
Frozen/Canned Mussel |
n = 1, PnTX-G (4 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Spain
|
Processed seafood samples |
Mussels in brine |
n = 1, PnTX-G (6 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Spain
|
Raw seafood samples (commerical) |
Mussel (Mytilus galloprovincialis) |
n = 1, PnTX-G (4 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Spain
|
Catalonia (NW Mediterranean Sea) |
Mussels and Oysters |
PnTX-G (2 to 60 μg/kg) |
NA |
(García-Altares et al., 2014) |
|
Spain
|
Galician Coastline |
Mussels (Mytilus galloprovincialis) |
PnTX-G (0.4 to 0.9 μg/kg) |
NA |
(Otero et al., 2019) |
|
Spain
|
Atlantic and Cantabrian Coasts |
Mussels (Mytilus galloprovincialis) |
PnTX-G (0 to 15 μg/kg) |
PnTX-A also detected but was but much lower levels.
<30% PnTX-G esterified |
(Lamas et al., 2019) |
|
Spain
|
Galician Coastline |
Mussels (Mytilus galloprovincialis) |
PnTX-G (3.1–7.7 μg/kg) |
NA |
(Varriale et al., 2021) |
|
Spain
|
Galician Coastline |
Mussels (Mytilus galloprovincialis) |
PnTX-G (2.3–3.1 μg/kg) |
3-22% PnTX-G esterified |
(Moreiras et al., 2020) |
|
Slovenia
|
Raw seafood samples (commerical) |
Flat Oysters (Ostrea edulis) |
n = 1, PnTX-G (4 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Slovenia
|
Processed seafood samples |
Mussels in tomato |
n = 2, PnTX-G (5 μg/kg and 12 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Slovenia
|
Processed seafood samples |
Frozen/Canned Mussel |
n = 1, PnTX-G (3 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Italy
|
Raw seafood samples (commerical) |
Clams (Venerupis decussata) |
n = 1, PnTX-G (4 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Italy
|
Processed seafood samples |
Frozen/Canned Mussel |
n = 1, PnTX-G (4 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Italy
|
Processed seafood samples |
Frozen/Canned Mussel |
n = 1, PnTX-G (3 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Italy
|
Sardinia, Tyrrhenian Sea |
Mussels (Mytilus galloprovincialis) |
n = 1 PnTX-G (6.8 μg/kg) |
NA |
(Varriale et al., 2021) |
|
Norway
|
Raw seafood samples (commercial) |
Blue mussels (Mytilus edulis) |
n = 2, PnTX-G (5.1 μg/kg and 3.2 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Norway
|
30 coastline locations |
Blue Mussel (Mytilus edulis) |
PnTX-G (0-115 μg/kg Mean 8.0 μg/kg) |
PnTX-A also detected but was 1% compared to PnTX-G |
(Rundberget et al., 2011) |
|
Ireland |
Raw seafood samples (commercial) |
Blue mussels (Mytilus edulis) |
n = 1, PnTX-G (4.6 μg/kg) |
NA |
(Rambla-Alegre et al., 2018) |
|
Scotland, Northern Ireland, Ireland, Italy, Norway and Portugal |
Unspecified Coastlines |
Mussels |
PnTX-A (0.9-2.6 μg/kg), PnTx-G (3.8-45.4 μg/kg) |
NA |
(Aráoz et al., 2020) |
|
Reports from Outside Europe |
Reports from Outside Europe |
Reports from Outside Europe |
Reports from Outside Europe |
Reports from Outside Europe |
Reports from Outside Europe |
|
Austrailia
|
Franklin Harbor, South Austrailia |
Pacific Oysters (Crassostrea gigas) |
PnTX-E, PnTX-F, & PnTX-G (no concentration reported) |
Trace amounts of PnTX-A and PnTX-G detected |
(Selwood et al., 2010) |
|
Austrailia
|
Franklin Harbor, South Austrailia |
Razor fish (Pinna bicolor) |
PnTX-A and PnTX-D (no concentration reported) |
Lower amounts of PnTX-E, PnTX-F, & PnTX-G detected (<10%) |
(Selwood et al., 2010) |
|
Canada |
Multiple locations across the eastern coast of Canada |
Mussels (Mytilus edulis) |
PnTX-G (0–83 μg/kg, Mean 12.0 μg/kg) |
PnTX-A also detected but was 3% compared to PnTx-G |
(McCarron et al., 2012) |
|
Chile |
Cooked seafood samples (commercial) |
Chilean mussels (Mytilus chilensis) |
PnTX-G (2.9-5.2 μg/kg) |
NA |
(Otero et al., 2020) |
|
Japan
|
Okinowa |
Prickly pen shell (Pinna muricata) |
PnTX-A (no concentration reported) |
NA |
(Uemura et al., 1995) |
|
Japan
|
Okinowa |
Prickly pen shell (Pinna muricata) |
PnTX-D (no concentration reported) |
NA |
(Chou et al., 1996) |
|
Japan
|
Okinowa |
Prickly pen shell (Pinna muricata) |
PnTX-B and PnTX-C (no concentration reported) |
NA |
(Takada et al., 2001) |
|
Mozambique |
Inhaca Island |
Flag pen shell (Atrina vexillum)
|
PnTX-G (14.3 μg/kg) |
46% PnTX-G esterified, PnTX-E and -F also detected |
(Tamele et al., 2022) |
|
Mozambique |
Inhaca Island |
Pearl oyster (Pinctada imbricata)
|
PnTX-G (2.4 μg/kg)
|
24% PnTX-G esterified,
PnTX-E and -F also detected |
(Tamele et al., 2022) |
|
Mozambique |
Inhaca Island |
Antique Ark (Anadara antiquata) |
PnTX-G (5.9 μg/kg) |
24% PnTX-G esterified,
PnTX-E and -F also detected |
(Tamele et al., 2022) |
|
New Zealand |
Rangaunu Harbor |
Pacific Oysters (Crassostrea gigas) |
PnTX-E and PnTX-F (no concentration reported) |
NA |
(Selwood et al., 2010) |
Impact of processing on pinnatoxin levels in shellfish
64. Information on the effects of industrial processing on the levels of PnTXs in shellfish is limited. In a study by Rambla-Alegre et al. (2018), fresh and processed shellfish samples from eight European countries (Italy, Portugal, Slovenia, Spain, Ireland, Norway, The Netherlands and Denmark) collected over two years, were tested for levels of PnTX-G and PnTX-A (Table 4). Processed samples underwent various industrial processes such as such as freezing, cooking, smoking and canning, as well as the mixing or addition of further ingredients such as sauces, pickles and brines.
65. PnTX-G was commonly detected in both fresh (39%) and processed (32%) samples. Processed mussels showed the highest concentrations of PnTX-G (12 µg/kg) compared to fresh mussels (5.1 µg/kg). LC-MS analysis found no PnTX-A in any of the fresh sample, but no testing data was available on the presence or levels of PnTX-A in the processed samples.
66. The authors suggest that some industrial process such as thermal processing of molluscs may result in a decrease in the total shellfish weight, via dehydration. In the absence of thermal degradation of the toxins, which they expect is low for lipophilic toxins, this could result in increased concentrations of toxins in the processed shellfish (Rambla-Alegre et al., 2018). The authors further noted that a study by Blanco et al. (2016) found industrial processes such as heat-sterilising (autoclaving) of mussels produced a significant reduction in the weight of canned mussels (average 25.5%). Steaming produced a reduction of around 30% in the canning process.
Pinnatoxins from other sources
Fish
67. Limited information is available on the impact of V. rugosum and/or PnTXs on organisms other than shellfish. Results from a 2022 study showed that Liza ramada (thin-lipped mullet) juveniles were able to predate on V. rugosum and that their tissues could be potentially contaminated by PnTX-G and portimine-A without impact on fish viability. Hence, L. ramada could potentially act as a vector for bioaccumulation in the food chain as well as transporting and dispersing V. rugosum cells with their migrations, potential increasing the area of habitation. No information on the potential human impact was available, but the authors noted that the levels of PnTX-G found in L. ramada tissues were in the same range as the critical sanitary threshold for PnTX-G in shellfish (23 µg/kg) (Bouquet et al., 2022).
Seaweed
68. PnTX-G has been identified in Saccharina latissimi (sugar kelp) from Norway by LC-MS analysis, with an estimated concentration of PnTX-G of 5.1 ± 0.4 µg/kg. These findings suggested that kelp or seaweeds might be habitats for the traditionally benthic V. rugosum in cold waters. Sugar kelp is commonly used in Japanese cuisine but no information was available on how levels of PnTXs found in the sugar kelp translate to potential human exposure (de la Iglesia et al., 2014).
Monitoring of Vulcanodinium rugosum
69. The peridinioid dinoflagellates V. rugosum are primarily benthic organisms, being found in marine sediment layers and have a relatively short motile stage, likely planktonic (Hernández-Becerril et al., 2013). Positive identification of V. rugosum is typically carried out by morphological identification under light and electron microscope; this has been used to positively identify V. rugosum in New Zealand, the Okinawan coastlines in Japan, the Mediterranean coastal bays and lagoons and the tropical waters of the Mexican Pacific coast (Table 5; Grzebyk et al., 2017; Hernández-Becerril et al., 2013; Rhodes et al., 2010; Satta et al., 2013; Selwood et al., 2010).
Table 5: Reports of detection of Vulcanodinium rugosum and pinnatoxin from seawater.
|
Country (Nearest) |
Sample Location |
V.rugosom detected?
|
PnTXs detected? |
Method of PnTX detection |
Reference |
|
European reports |
European reports |
European reports |
European reports |
European reports |
European reports |
|
Ireland |
Lough Hyne |
Not detected |
PnTX-G |
LC-MS of Active sampling + SPATT Disks |
(McCarthy et al., 2015) |
|
France |
6 lagoons along the Languedoc-Roussillon coast |
Yes
|
No testing for toxins was performed |
|
(Grzebyk et al., 2017) |
|
France |
Ingril Lagoon |
Yes
|
PnTX-G |
LC-MS/MS of from culture extract |
(Abadie et al., 2018, 2016, 2015) |
|
Norway |
30 coastline locations |
No |
PnTX-G
|
LC-MS of of SPATT Disks |
(Rundberget et al., 2011) |
|
Spain |
Fangar bay, Catalonia |
Yes
|
No testing for toxins was performed |
NA |
(Satta et al., 2013) |
|
Reports from Outside Europe |
Reports from Outside Europe |
Reports from Outside Europe |
Reports from Outside Europe |
Reports from Outside Europe |
Reports from Outside Europe |
|
Australia |
Franklin Harbor, South Austrailia |
No |
PnTX-G, PnTX-E & PnTX-F |
LC-MS of Sediment and water samples |
(Selwood et al., 2010) |
|
Australia |
Franklin Harbor, South Austrailia |
Yes
|
PnTX-G, PnTX-E, PnTX-F,
Trace amounts PnTX-A |
LC-MS of water samples from culture of clonal isolate |
(Rhodes et al., 2011a) |
|
Chile |
16 stations along coastal shores of Chile |
No
|
PnTX-G |
LC-MS of SPATT Bags |
(Möller et al., 2022) |
|
Cuba |
Cienfuegos Bay |
Yes
|
PnTX-E & PnTX-F
Trace amounts PnTX-D, PnTX-G |
LC-MS of water samples |
(Moreira-González et al., 2021) |
|
Qatar
|
4 Sampling sites east of the Qatar Peninsula |
Yes
|
PnTX-G & PnTX-H |
Enzyme linked immuno-sorbent assay (ELISA) and LC-MS/MS) |
(Al Muftah et al., 2016) |
|
Japan |
Ishigaki-Jima, Okinawa |
Yes
|
PnTX-G |
LC-MS of water samples from culture of clonal isolate |
(Smith et al., 2011) |
|
Japan |
Okinawa |
Yes
|
NA |
NA |
(Rhodes et al., 2011b) |
|
Mexico |
Offshore of Lazaro Cárde-nas, Michoacán, Mexico |
Yes |
No testing for toxins was performed |
NA |
(Hernández-Becerril et al., 2013) |
|
Thailand |
Gulf of Thailand |
Yes
|
No testing for toxins was performed |
NA |
(Fu et al., 2021) |
|
New Zealand |
Rangaunu Harbor |
Yes
|
PnTX-E & PnTX-F,
Trace amounts of PnTX-D |
LC-MS of culture of clonal isolate |
(Rhodes et al., 2011b, 2010) |
|
South China Sea |
No Detail |
Yes
|
PnTX-H
|
LC-MS of cultures of clonal isolate |
(Selwood et al., 2014) |
70. Applying a microscopy approach to wider surveillance programs and monitoring for microalgal toxins is laborious and time-consuming, and generally poorly adapted to the identification and collection of small toxin-producing organisms such as V. rugosum (Roué et al., 2018). More recent technologies have aided greater detection and monitoring of toxigenic species. Passive sampling solid phase adsorption toxin tracking (SPATT) disks use porous synthetic resins which passively adsorb toxins produced by microalgae dissolved in the seawater (MacKenzie et al., 2004). Similarly, improvements in active sampling methods using large-scale solid-phase extraction (SPE) method have allowed the large-scale pumping of seawater through a series of filtration devices and through a cartridge containing the resin in the goal of detecting microalgal toxins (Rundberget et al., 2007). Combined with techniques such as LC/MS, these approaches have been successfully used to detect the presence of PnTX-G off the coast of Ireland, Norway and Chile. In these cases, the causative V. rugosom species was not positively identified, but their presence was inferred as, to date, no other PnTX-producing organism has been identified (Table 5; McCarthy et al., 2015; Möller et al., 2022; Rundberget et al., 2011).
Environmental factors affecting V. rugosum and pinnatoxin occurrence
71. The marine environment has a significant impact on the growth and persistence of algal colonies. Once the causative organism is present, favourable temperature ranges, chemical environments and nutrient sources can lead to the accumulation of potentially harmful algae, such as V. rugosum and can lead to harmful algal blooms (HABs).
Impact of Sea Temperature on V. rugosum
72. Initial reports of V. rugosum detected in the waters of Southern Australia, Mexico and French Mediterranean lagoons seemed to suggest that the species preferred warmer waters. This was supported by surveillance data at the Ingril Lagoon over a 4 year period (2009 to 2012) which showed increased the occurrence of PnTXs in shellfish during June-November period with the peaks during the Summer months, although V. rugosum growth was not examined (Hess et al., 2013). However, discovery of substantial pinnatoxins contamination of shellfish from a range of Norwegian costal location also suggests that V. rugosum is able to thrive in colder environments (Rundberget et al., 2011).
73. Cultures of clonal V. rugosum grew optimally at 25°C, but cysts were the predominant life stage at temperatures < 20°C or > 30°C (Rhodes et al., 2010). A study by Abadie et al. (2016) systematically investigated V. rugosum growth in an enriched natural seawater medium, under a range of temperatures and salinities. The highest growth rates and cell densities occurred at 25°C (0.21 to 0.39/d) and 30 °C (0.16 to 0.26/d), suggesting that V. rugosum prefers warmer temperatures. The V. rugosum strain, isolated from a French Mediterranean lagoon in 2010, grew at 20°C, but at a relatively low rate (0.13/d) and with a long lag phase (18 days). At temperatures ≤ 15°C and ≥ 35°C V. rugosum did not show any growth (Abadie et al., 2016).
74. Water samples taken over a 12-month period from March 2012 to March 2013 at the Ingril lagoon, France, showed a high correlation between V. rugosum abundance (cell/L) in the water column and the water temperature (Spearman coefficient: r = 0.658, p < 0.01). The dinoflagellate was observed as early in season as March (temperature of 14°C) but at low concentration (4 cell/L), while densities higher than 50 cell/L were observed June to September (temperature 21°C to 29°C) (Abadie et al., 2018).
Impact of Salinity on V. rugosum
75. Abadie et al. (2016) also examined the effect of salinity on growth and concluded that the most favourable salinity conditions for growth ranged between 25 and 40 (units not specified; seawater is typically around 35). Under these conditions, growth rate ranged between 0.21/d and 0.39/d and cell density between 1952 cells/mL and 4252 cells/mL. At lower salinity of 20, cell growth was inhibited and only observed at higher temperature (30°C) conditions, with low cell yield. V. rugosum did not grow at a salinity of 15. These results suggested that V. rugosum has an optimal combination for growth (0.39 ± 0.11 per day) of a temperature of 25°C and a salinity of 40 (Abadie et al., 2016).
76. The data also indicated that salinity was positively correlated to V. rugosum abundance in natural marine environments. Samples taken over a 12-month period from March 2012 to March 2013 at the Ingril lagoon, France, showed a correlation between V. rugosum abundance (cell/L) with water salinity (Spearman coefficient: r = 0.526, p < 0.01). The observations from these environmental studies were in agreement with the results under laboratory conditions, the maximum V. rugosum concentration found in water columns (995 cell/L) occurred at a temperature of 23.9°C and a salinity of 40.5 (Abadie et al., 2018).
The impact of climate change on the risk of V. rugosum and pinnatoxin in UK waters
77. Warmer ocean temperatures and changes in local climate trends have been associated with intensifying HABs, promoting algal growth and broadening the seasonal timeframe in which these toxic algae can accumulate (Gobler et al., 2017; Moore et al., 2009). Changes in temperature in traditionally cooler waters as a result of climate change may result in HABs migrating pole-ward with progressive warming, a hypothesis that has been affirmed by several case studies (Gobler et al., 2017; Griffith et al., 2019).
78. The effect of ocean warming on the formation of HABs is particularly relevant for cyst-forming dinoflagellates. Many phytoplankton species, including V. rugosum, spend extended periods in a benthic resting stage. These stages, called cysts, germinate in response to the combination of favourable temperature, oxygen-availability, and release from dormancy. Simulations of changing ocean environments have shown complex interactions with the different life stages of dinoflagellate species, and suggested that as marine environments continue to warm, the geographical distribution and seasonal timing of these algal blooms may be affected (Brosnahan et al., 2020).
79. This concern was also expressed by ANSES in their 2019 opinion on PnTX monitoring: “Given that global warming is making ecophysiological conditions more favourable to the development of V. rugosum, vigilance should be maintained along the Atlantic coast” (ANSES, 2019)
Summary and conclusions
80. Pinnatoxins (PnTXs) are produced by the algae V. rugosum and bioaccumulate in filter feeding shellfish such as mussels and clams, and hence, are of potential risk to human health.
81. No human cases of intoxication from PnTXs have been reported to date. However, the available data showed potent acute toxicity of PnTXs in mice, both by oral and i.p injection, blocking nerve signals by inhibiting ACh-evoked nAChRs activity.
82. To date, there are no published data that indicate the presence of PnTXs in UK shellfish, but PnTXs has been found in shellfish from Norway, France and Northern Spain. Given these occurrences, and the temperature profile of V. rugosum algae it is possible, that PnTx is already or will be present in UK waters.
Questions for the Committee
i. Do the Committee think there is a public health risk related to PnTXs?
ii. Do the Committee think there is sufficient toxicological and occurrence data to carry out a risk assessment on PnTXs in shellfish from UK waters?
iii. Do the Committee consider it necessary to include PnTXs in the FSA monitoring programme for England and Wales?
iv. Do the Committee have any comments on whether increasing temperatures in UK waters would lead to an increase occurrence of V. rugosum and PnTXs contamination of UK shellfish.
v. Do the Committee have any other comments?
Secretariat
July 2023
Abbreviations
|
ABFI |
The Scottish Association of Marine Science |
|
ACh |
Acetylcholine |
|
ANSES |
The French Agency for Food, Environmental and Occupational Health & Safety |
|
ARfD |
Acute Reference Dose |
|
BMDL |
Benchmark Dose Lower Confidence Limit |
|
b.w. |
Bodyweight |
|
CEFAS |
The Centre for Environment, Fisheries and Aquaculture Science |
|
CI |
Cyclic Imine |
|
CONTAM |
The EFSA Panel on Contaminants in the Food Chain |
|
COT |
Committee on Toxicology |
|
CMAP |
Compound Muscle Action Potentials |
|
DG |
Digestive Gland |
|
EDL |
Extensor Digitorum Longus (muscle) |
|
EFSA |
The European Food Safety Authority |
|
FSA |
Food Standards Agency |
|
HAB |
Harmful Algal Bloom |
|
IC50 |
Inhibitory concentration (50%): The concentration of a substance required to block 50% of a given response |
|
IFREMER |
National Institute for Ocean Science in France |
|
i.p. |
intraperitoneal |
|
i.v. |
Intravenous |
|
KCl |
Potassium Chloride |
|
LC-MS/MS |
Liquid Chromatography with Mass Spectrometry |
|
LD50/99 |
Lethal Dose (50%) / Lethal Dose (99%): The dose of a substance that kills 50 / 99 % of the test sample |
|
MLA |
Methyllycaconitine |
|
MTD |
Maximum Tolerated Dose |
|
nAChRs |
Nicotinic Acetylcholine Receptors |
|
NOAEL |
No-Observed-Adverse-Effect Levels |
|
PnTX |
Pinnatoxin |
|
PSP |
Paralytic Shellfish Poisoning |
|
SAMS |
The Scottish Association of Marine Science |
|
SEM |
Standard Error of the Mean |
|
SPATT |
Solid Phase Adsorption Toxin Tracking |
|
SPE |
Solid-Phase Extraction |
|
SuG |
Superficial Grey Layer of the Superior Colliculus |
|
TDI |
Tolerable Daily Intake |
|
UF |
Uncertainty factor |
|
WG |
Working Group |
|
WG |
Working Group |
|
YFP |
Yellow Fluorescent Protein |
References
Abadie, E., Chiantella, C., Crottier, A., Rhodes, L., Masseret, E., Berteaux, T., Laabir, M., 2018. What are the main environmental factors driving the development of the neurotoxic dinoflagellate Vulcanodinium rugosum in a Mediterranean ecosystem (Ingril lagoon, France)? Harmful Algae 75, 75–86. https://doi.org/10.1016/j.hal.2018.03.012
Abadie, E., Kaci, L., Berteaux, T., Hess, P., Sechet, V., Masseret, E., Rolland, J.L., Laabir, M., 2015. Effect of Nitrate, Ammonium and Urea on Growth and Pinnatoxin G Production of Vulcanodinium rugosum. Marine Drugs 13, 5642–5656. https://doi.org/10.3390/md13095642
Abadie, E., Muguet, A., Berteaux, T., Chomérat, N., Hess, P., Roque D’OrbCastel, E., Masseret, E., Laabir, M., 2016. Toxin and Growth Responses of the Neurotoxic Dinoflagellate Vulcanodinium rugosum to Varying Temperature and Salinity. Toxins 8, 136.https://doi.org/10.3390/toxins8050136
Al Muftah, A., Selwood, A.I., Foss, A.J., Al-Jabri, H.M.S.J., Potts, M., Yilmaz, M., 2016. Algal toxins and producers in the marine waters of Qatar, Arabian Gulf. Toxicon 122, 54–66. https://doi.org/10.1016/j.toxicon.2016.09.016
ANSES, 2019. OPINION of the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) on the assessment of the health risks associated with pinnatoxins in shellfish.
ANSES-Universite de Trieste-Cnr, 2014. Pinnatoxines en lien avec l’espece Vulcanodinium rugosum (II) Partie 2: Etudes sur la toxicite des pinnatoxines et d’extraits de Vulcanodinium rugosum.
Aráoz, R., Barnes, P., Sechet, V., Delepierre, M., Zinn-Justin, S., Molgó, J., Zakarian, A., Hess, P., Servent, D., 2020. Cyclic imine toxins survey in coastal European shellfish samples: Bioaccumulation and Mode of Action of 28-O-palmitoyl ester of pinnatoxin-G. First Report of portimine-A bioaccumulation. Harmful Algae 98, 101887. https://doi.org/10.1016/j.hal.2020.101887
Araoz, R., Servent, D., Molgó, J., Iorga, B.I., Fruchart-Gaillard, C., Benoit, E., Gu, Z., Stivala, C., Zakarian, A., 2011. Total Synthesis of Pinnatoxins A and G and Revision of the Mode of Action of Pinnatoxin A. J. Am. Chem. Soc. 133, 10499–10511. https://doi.org/10.1021/ja201254c
Arnich, N., Abadie, E., Delcourt, N., Fessard, V., Fremy, J.-M., Hort, V., Lagrange, E., Maignien, T., Molgó, J., Peyrat, M.-B., Vernoux, J.-P., Mattei, C., 2020. Health risk assessment related to pinnatoxins in French shellfish. Toxicon 180, 1–10. https://doi.org/10.1016/j.toxicon.2020.03.007
Benoit, E., Couesnon, A., Lindovsky, J., Iorga, B.I., Aráoz, R., Servent, D., Zakarian, A., Molgó, J., 2019. Synthetic Pinnatoxins A and G Reversibly Block Mouse Skeletal Neuromuscular Transmission In Vivo and In Vitro. Marine Drugs 17, 306. https://doi.org/10.3390/md17050306
Blanco, J., Arévalo, F., Correa, J., Porro, M.C., Cabado, A.G., Vieites, J.M., Moroño, A., 2016. Effect of the industrial canning on the toxicity of mussels contaminated with diarrhetic shellfish poisoning (DSP) toxins. Toxicon 112, 1–7. https://doi.org/10.1016/j.toxicon.2016.01.061
Bouquet, A., Perdrau, M.A., Laabir, M., Foucault, E., Chomérat, N., Rolland, J.L., Abadie, E., 2022. Liza ramada Juveniles after Exposure to the Toxic Dinoflagellate Vulcanodinium rugosum: Effects on Fish Viability, Tissue Contamination and Microalgae Survival after Gut Passage. Toxins (Basel) 14, 401. https://doi.org/10.3390/toxins14060401
Bourne, Y., Sulzenbacher, G., Radić, Z., Aráoz, R., Reynaud, M., Benoit, E., Zakarian, A., Servent, D., Molgó, J., Taylor, P., Marchot, P., 2015. Marine Macrocyclic Imines, Pinnatoxins A and G: Structural Determinants and Functional Properties to Distinguish Neuronal α7 from Muscle α12βγδ nAChRs. Structure 23, 1106–1115. https://doi.org/10.1016/j.str.2015.04.009
Brosnahan, M.L., Fischer, A.D., Lopez, C.B., Moore, S.K., Anderson, D.M., 2020. Cyst-forming dinoflagellates in a warming climate. Harmful Algae, Climate change and harmful algal blooms 91, 101728. https://doi.org/10.1016/j.hal.2019.101728
CEFAS, 2014. Research to Support the Development of a Monitoring Programme for New or Emerging Marine Biotoxins in Shellfish in UK Waters. The Centre for Environment, Fisheries and Aquaculture Science.
Chou, T., Haino, T., Kuramoto, M., Uemura, D., 1996. Isolation and structure of pinnatoxin D, a new shellfish poison from the okinawan bivalve Pinna muricata. Tetrahedron Letters 37, 4027–4030. https://doi.org/10.1016/0040-4039(96)00753-8
Cuddihy, S.L., Drake, S., Harwood, D.T., Selwood, A.I., McNabb, P.S., Hampton, M.B., 2016. The marine cytotoxin portimine is a potent and selective inducer of apoptosis. Apoptosis 21, 1447–1452. https://link.springer.com/article/10.1007/s10495-016-1302-x
de la Iglesia, P., del Río, V., Diogène, J., 2014. The sugar kelp Saccharina latissima is a potential source of the emerging toxin, Pinnatoxin-G, in cold waters, in: Proceedings of the 16th International Conference on Harmful Algae. Presented at the International Society for the Study of Harmful Algae, Wellington, New Zealand.
Delcourt, N., Lagrange, E., Abadie, E., Fessard, V., Frémy, J.-M., Vernoux, J.-P., Peyrat, M.-B., Maignien, T., Arnich, N., Molgó, J., Mattei, C., 2019. Pinnatoxins’ Deleterious Effects on Cholinergic Networks: From Experimental Models to Human Health. Marine Drugs 17, 425. https://doi.org/10.3390/md17070425
EFSA, 2010. Scientific Opinion on marine biotoxins in shellfish – Cyclic imines (spirolides, gymnodimines, pinnatoxins and pteriatoxins) | EFSA.
FAO, 2004. Report of the Joint FAO/IOC/WHO ad hoc Expert Consultation on Biotoxins in Bivalve Molluscs - Technical report.
Fu, Z., Piumsomboon, A., Punnarak, P., Uttayarnmanee, P., Leaw, C.P., Lim, P.T., Wang, A., Gu, H., 2021. Diversity and distribution of harmful microalgae in the Gulf of Thailand assessed by DNA metabarcoding. Harmful Algae 106, 102063. https://doi.org/10.1016/j.hal.2021.102063
García-Altares, M., Casanova, A., Bane, V., Diogène, J., Furey, A., de la Iglesia, P., 2014. Confirmation of pinnatoxins and spirolides in shellfish and passive samplers from Catalonia (Spain) by liquid chromatography coupled with triple quadrupole and high-resolution hybrid tandem mass spectrometry. Mar Drugs 12, 3706–3732. https://doi.org/10.3390/md12063706
Geiger, M., Desanglois, G., Hogeveen, K., Fessard, V., Leprêtre, T., Mondeguer, F., Guitton, Y., Hervé, F., Séchet, V., Grovel, O., Pouchus, Y.-F., Hess, P., 2013. Cytotoxicity, Fractionation and Dereplication of Extracts of the Dinoflagellate Vulcanodinium rugosum, a Producer of Pinnatoxin G. Marine Drugs 11, 3350. https://doi.org/10.3390/md11093350
Gobler, C.J., Doherty, O.M., Hattenrath-Lehmann, T.K., Griffith, A.W., Kang, Y., Litaker, R.W., 2017. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proceedings of the National Academy of Sciences 114, 4975–4980. https://doi.org/10.1073/pnas.1619575114
Griffith, A.W., Doherty, O.M., Gobler, C.J., 2019. Ocean warming along temperate western boundaries of the Northern Hemisphere promotes an expansion of Cochlodinium polykrikoides blooms. Proceedings of the Royal Society B: Biological Sciences 286, 20190340. https://doi.org/10.1098/rspb.2019.0340
Grzebyk, D., Audic, S., Lasserre, B., Abadie, E., de Vargas, C., Bec, B., 2017. Insights into the harmful algal flora in northwestern Mediterranean coastal lagoons revealed by pyrosequencing metabarcodes of the 28S rRNA gene. Harmful Algae 68, 1–16. https://doi.org/10.1016/j.hal.2017.06.003
Hellyer, S.D., Indurthi, D., Balle, T., Runder-Varga, V., Selwood, A.I., Tyndall, J.D.A., Chebib, M., Rhodes, L., Kerr, D.S., 2015. Pinnatoxins E, F and G target multiple nicotinic receptor subtypes. Journal of Neurochemistry 135, 479–491. https://doi.org/10.1111/jnc.13245
Hellyer, S.D., Selwood, A.I., Rhodes, L., Kerr, D.S., 2013. Neuromuscular blocking activity of pinnatoxins E, F and G. Toxicon 76, 214–220. https://doi.org/10.1016/j.toxicon.2013.10.009
Hellyer, S.D., Selwood, A.I., Rhodes, L., Kerr, D.S., 2011a. Marine algal pinnatoxins E and F cause neuromuscular block in an in vitro hemidiaphragm preparation. Toxicon 58, 693–699.https://doi.org/10.1016/j.toxicon.2011.09.006
Hellyer, S.D., Selwood, A.I., Rhodes, L., Kerr, D.S., 2011b. Marine algal pinnatoxins E and F cause neuromuscular block in an in vitro hemidiaphragm preparation. Toxicon 58, 693–699. https://doi.org/10.1016/j.toxicon.2011.09.006
Hellyer, S.D., Selwood, A.I., van Ginkel, R., Munday, R., Sheard, P., Miles, C.O., Rhodes, L., Kerr, D.S., 2014. In vitro labelling of muscle type nicotinic receptors using a fluorophore-conjugated pinnatoxin F derivative. Toxicon 87, 17–25. https://doi.org/10.1016/j.toxicon.2014.05.013
Hernández-Becerril, D.U., Rodríguez-Palacio, M.C., Lozano-Ramírez, C., 2013. Morphology and life stages of the potentially pinnatoxin-producing thecate dinoflagellate Vulcanodinium rugosum from the tropical Mexican Pacific. Botanica Marina 56, 535–540.https://doi.org/10.1515/bot-2013-0079
Hess, P., Abadie, E., Hervé, F., Berteaux, T., Séchet, V., Aráoz, R., Molgó, J., Zakarian, A., Sibat, M., Rundberget, T., Miles, C.O., Amzil, Z., 2013. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from Ingril, a French Mediterranean lagoon. Toxicon 75, 16–26. https://doi.org/10.1016/j.toxicon.2013.05.001
Hess, P., Nguyen, L., Aasen, J., Keogh, M., Kilcoyne, J., McCarron, P., Aune, T., 2005. Tissue distribution, effects of cooking and parameters affecting the extraction of azaspiracids from mussels, Mytilus edulis, prior to analysis by liquid chromatography coupled to mass spectrometry. Toxicon 46, 62–71. https://doi.org/10.1016/j.toxicon.2005.03.010
Lamas, J.P., Arévalo, F., Moroño, Á., Correa, J., Muñíz, S., Blanco, J., 2019. Detection and Spatio-Temporal Distribution of Pinnatoxins in Shellfish from the Atlantic and Cantabrian Coasts of Spain. Toxins 11, 340. https://doi.org/10.3390/toxins11060340
MacKenzie, L., Beuzenberg, V., Holland, P., McNabb, P., Selwood, A., 2004. Solid phase adsorption toxin tracking (SPATT): a new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 44, 901–918. https://doi.org/10.1016/j.toxicon.2004.08.020
McCarron, P., Kilcoyne, J., Hess, P., 2008. Effects of cooking and heat treatment on concentration and tissue distribution of okadaic acid and dinophysistoxin-2 in mussels (Mytilus edulis). Toxicon 51, 1081–1089. https://doi.org/10.1016/j.toxicon.2008.01.009
McCarron, P., Rourke, W.A., Hardstaff, W., Pooley, B., Quilliam, M.A., 2012. Identification of Pinnatoxins and Discovery of Their Fatty Acid Ester Metabolites in Mussels (Mytilus edulis) from Eastern Canada. J. Agric. Food Chem. 60, 1437–1446. https://doi.org/10.1021/jf204824s
McCarthy, M., Bane, V., García-Altares, M., van Pelt, F.N.A.M., Furey, A., O’Halloran, J., 2015. Assessment of emerging biotoxins (pinnatoxin G and spirolides) at Europe’s first marine reserve: Lough Hyne. Toxicon 108, 202–209. https://doi.org/10.1016/j.toxicon.2015.10.007
Molgó, J., Marchot, P., Aráoz, R., Benoit, E., Iorga, B.I., Zakarian, A., Taylor, P., Bourne, Y., Servent, D., 2017. Cyclic imine toxins from dinoflagellates: a growing family of potent antagonists of the nicotinic acetylcholine receptors. J Neurochem 142 Suppl 2, 41–51.https://doi.org/10.1111/jnc.13995
Möller, K., Pinto-Torres, M., Mardones, J.I., Krock, B., 2022. Distribution of phycotoxins in Última Esperanza Province during the PROFAN expedition 2019. Progress in Oceanography 206, 102851. https://doi.org/10.1016/j.pocean.2022.102851
Moore, S.K., Mantua, N.J., Hickey, B.M., Trainer, V.L., 2009. Recent trends in paralytic shellfish toxins in Puget Sound, relationships to climate, and capacity for prediction of toxic events. Harmful Algae 8, 463–477. https://doi.org/10.1016/j.hal.2008.10.003
Moreira-González, A.R., Comas-González, A., Valle-Pombrol, A., Seisdedo-Losa, M., Hernández-Leyva, O., Fernandes, L.F., Chomérat, N., Bilien, G., Hervé, F., Rovillon, G.A., Hess, P., Alonso-Hernández, C.M., Mafra, L.L., 2021. Summer bloom of Vulcanodinium rugosum in Cienfuegos Bay (Cuba) associated to dermatitis in swimmers. Science of The Total Environment 757, 143782. https://doi.org/10.1016/j.scitotenv.2020.143782
Moreiras, G., Leão, J.M., Gago-Martínez, A., 2020. Analysis of Cyclic Imines in Mussels (Mytilus galloprovincialis) from Galicia (NW Spain) by LC-MS/MS. International Journal of Environmental Research and Public Health 17, 281. https://doi.org/10.3390/ijerph17010281
Munday, R., Selwood, A.I., Rhodes, L., 2012. Acute toxicity of pinnatoxins E, F and G to mice. Toxicon 60, 995–999. https://doi.org/10.1016/j.toxicon.2012.07.002
Otero, P., Miguéns, N., Rodríguez, I., Botana, L.M., 2019. LC-MS/MS Analysis of the Emerging Toxin Pinnatoxin-G and High Levels of Esterified OA Group Toxins in Galician Commercial Mussels. Toxins (Basel) 11, E394. https://doi.org/10.3390/toxins11070394
Otero, P., Vale, C., Boente-Juncal, A., Costas, C., Louzao, M.C., Botana, L.M., 2020. Detection of Cyclic Imine Toxins in Dietary Supplements of Green Lipped Mussels (Perna canaliculus) and in Shellfish Mytilus chilensis. Toxins 12, 613. https://doi.org/10.3390/toxins12100613
Rambla-Alegre, M., Miles, C.O., de la Iglesia, P., Fernandez-Tejedor, M., Jacobs, S., Sioen, I., Verbeke, W., Samdal, I.A., Sandvik, M., Barbosa, V., Tediosi, A., Madorran, E., Granby, K., Kotterman, M., Calis, T., Diogene, J., 2018. Occurrence of cyclic imines in European commercial seafood and consumers risk assessment. Environmental Research 161, 392–398.https://doi.org/10.1016/j.envres.2017.11.028
Rhodes, L., Smith, K., Selwood, A., McNabb, P., Molenaar, S., Munday, R., Wilkinson, C., Hallegraeff, G., 2011a. Production of pinnatoxins E, F and G by scrippsielloid dinoflagellates isolated from Franklin Harbour, South Australia. New Zealand Journal of Marine and Freshwater Research 45, 703–709. https://doi.org/10.1080/00288330.2011.586041
Rhodes, L., Smith, K., Selwood, A., McNabb, P., Munday, R., Suda, S., Molenaar, S., Hallegraeff, G., 2011b. Dinoflagellate Vulcanodinium rugosum identified as the causative organism of pinnatoxins in Australia, New Zealand and Japan. Phycologia 50, 624–628. https://doi.org/10.2216/11-19.1
Rhodes, L., Smith, K., Selwood, A., McNabb, P., van Ginkel, R., Holland, P., Munday, R., 2010. Production of pinnatoxins by a peridinoid dinoflagellate isolated from Northland, New Zealand. Harmful Algae 9, 384–389. https://doi.org/10.1016/j.hal.2010.01.008
Roué, M., Darius, H.T., Chinain, M., 2018. Solid Phase Adsorption Toxin Tracking (SPATT) Technology for the Monitoring of Aquatic Toxins: A Review. Toxins (Basel) 10, 167. https://doi.org/10.3390/toxins10040167
Rundberget, T., Aasen, J.A.B., Selwood, A.I., Miles, C.O., 2011. Pinnatoxins and spirolides in Norwegian blue mussels and seawater. Toxicon 58, 700–711. https://doi.org/10.1016/j.toxicon.2011.08.008
Rundberget, T., Sandvik, M., Larsen, K., Pizarro, G.M., Reguera, B., Castberg, T., Gustad, E., Loader, J.I., Rise, F., Wilkins, A.L., Miles, C.O., 2007. Extraction of microalgal toxins by large-scale pumping of seawater in Spain and Norway, and isolation of okadaic acid and dinophysistoxin-2. Toxicon 50, 960–970. https://doi.org/10.1016/j.toxicon.2007.07.003
Satta, C.T., Anglès, S., Lugliè, A., Guillén, J., Sechi, N., Camp, J., Garcés, E., 2013. Studies on dinoflagellate cyst assemblages in two estuarine Mediterranean bays: A useful tool for the discovery and mapping of harmful algal species. Harmful Algae 24, 65–79. https://doi.org/10.1016/j.hal.2013.01.007
Selwood, A.I., Miles, C.O., Wilkins, A.L., Ginkel, R. van, Munday, R., Rise, F., McNabb, P., 2010. Isolation, Structural Determination and Acute Toxicity of Pinnatoxins E, F and G [WWW Document]. ACS Publications. https://doi.org/10.1021/jf100267a
Selwood, A.I., Wilkins, A.L., Munday, R., Gu, H., Smith, K.F., Rhodes, L.L., Rise, F., 2014. Pinnatoxin H: a new pinnatoxin analogue from a South China Sea Vulcanodinium rugosum isolate. Tetrahedron Letters 55, 5508–5510. https://doi.org/10.1016/j.tetlet.2014.08.056
Servent, D., Malgorn, C., Bernes, M., Gil, S., Simasotchi, C., Hérard, A.-S., Delzescaux, T., Thai, R., Barbe, P., Keck, M., Beau, F., Zakarian, A., Dive, V., Molgó, J., 2021. First evidence that emerging pinnatoxin-G, a contaminant of shellfish, reaches the brain and crosses the placental barrier. Science of The Total Environment 790, 148125. https://doi.org/10.1016/j.scitotenv.2021.148125
Smith, K.F., Rhodes, L.L., Suda, S., Selwood, A.I., 2011. A dinoflagellate producer of pinnatoxin G, isolated from sub-tropical Japanese waters. Harmful Algae 10, 702–705. https://doi.org/10.1016/j.hal.2011.05.006
Sosa, S., Pelin, M., Cavion, F., Hervé, F., Hess, P., Tubaro, A., 2020. Acute Oral Toxicity of Pinnatoxin G in Mice. Toxins (Basel) 12, 87. https://doi.org/10.3390/toxins12020087
Takada, N., Umemura, N., Suenaga, K., Chou, T., Nagatsu, A., Haino, T., Yamada, K., Uemura, D., 2001. Pinnatoxins B and C, the most toxic components in the pinnatoxin series from the Okinawan bivalve Pinna muricata. Tetrahedron letters 42, 3491–3494. https://doi.org/10.1016/S0040-4039(01)00480-4
Tamele, I.J., Timba, I., Vasconcelos, V., Costa, P.R., 2022. First Report of Pinnatoxins in Bivalve Molluscs from Inhaca Island (South of Mozambique)—South of the Indian Ocean. Journal of Marine Science and Engineering 10, 1215. https://doi.org/10.3390/jmse10091215
Uemura, D., Chou, T., Haino, T., Nagatsu, A., Fukuzawa, S., Zheng, S., Chen, H., 1995. Pinnatoxin A: a toxic amphoteric macrocycle from the Okinawan bivalve Pinna muricata. ACS Publications. https://doi.org/10.1021/ja00108a043
Varriale, F., Tartaglione, L., Cinti, S., Milandri, A., Dall’Ara, S., Calfapietra, A., Dell’Aversano, C., 2021. Development of a data dependent acquisition-based approach for the identification of unknown fast-acting toxins and their ester metabolites. Talanta 224, 121842. https://doi.org/10.1016/j.talanta.2020.121842
Zendong, Z., Herrenknecht, C., Abadie, E., Brissard, C., Tixier, C., Mondeguer, F., Sechet, V., Amzil, Z., Hess, P., 2014. Extended evaluation of polymeric and lipophilic sorbents for passive sampling of marine toxins. Toxicon 91, 57–68. https://doi.org/10.1016/j.toxicon.2014.03.010
Zheng, S., Huang, F., Chen, S., Tan, X., Zuo, J., Peng, J., Xie, R., 1990. The isolation and bioactivities of pinnatoxin. Chin. J. Mar. Drugs 9, 33–35.
TOX/2023/37 Annex A
Literature search on pinnatoxins
A literature search was conducted as part of this discussion paper, using the following databases and criteria.
The Database search included searches of PubMed, Science Direct and Google Scholar for the terms “Vulcanodinium rugosum” and “Pinnatoxin”. All papers containing the relevant terms up to and including the 1st March 2023 were considered.
|
Website |
Vulcanodinium rugosum No start date, End date: 1st March 2023 |
Pinnatoxin No start date, End date: 1st March 2023 |
|
PUBMED |
23 |
67 |
|
Science Direct* |
45 |
138 |
|
Google Scholar |
347 |
1,320 |
*Filter settings: Limited to “Research articles”
Exclusion Criteria: Papers were excluded if they did not relate directly to pinnatoxin, focused on the manufacture and chemical synthesis of pinnatoxin or focused on analytical method development for detection and analysis of PnTXs. Purely in silico studies of toxicology or mechanisms of action are also not considered.
TOX/2023/37 Annex B
Activity of pinnatoxins against nicotinic acetylcholine receptors
Inhibition constants (IC50, nM) for the action of PnTX-analogues on ACh-evoked nicotinic currents, recorded from oocytes expressing the human neuronal α7, α4β2 or Torpedo nAChR subtypes (various subunit stoichiometries for the α4β2).Values are Mean with either SEM (±) or 95% confidence intervals (indicated in parentheses) (Adapted from Molgó et al., 2017).
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
a Data obtained with (α4)3(β2)2; b Data obtained with (α4)2(β2)3.
References
Molgó, J., Marchot, P., Aráoz, R., Benoit, E., Iorga, B.I., Zakarian, A., Taylor, P., Bourne, Y., Servent, D., 2017. Cyclic imine toxins from dinoflagellates: a growing family of potent antagonists of the nicotinic acetylcholine receptors. J Neurochem 142 Suppl 2, 41–51. https://doi.org/10.1111/jnc.13995